Welcome to Part-Two of our Indoor Air Quality series highlighting the latest studies released about COVID-19 as it relates to IAQ…
Catch up on Part-One here if you missed it.
The introduction of the novel coronavirus in early 2020 changed our lives forever. It is generally a once-in-a-century occurrence for the entire world to participate in the shared experience of a Pandemic. Initially, scientists worked tirelessly to produce vaccines to combat COVID-19. Now, 19-months after the CDC officially declared that SARS-Cov-2 was a Pandemic, we have compiled data from independent studies about Covid-19 and its relationship with indoor air quality.
Compared to the bleak outlook in the beginning, society now has a better understanding of COVID-19 and how it works. However, there are still many unanswered questions. Considering how easily this novel coronavirus is transmitted, especially with the new variants in indoor settings, it is more important than ever to consider insight gleaned from scientific studies such as what we highlight here.
🔍 Today, we are going to examine four case studies that were recently released:
- “Spread of SARS-CoV-2 in hospital areas”
- “Airborne SARS-CoV-2 surveillance in hospital environment using high-flowrate air samplers and its comparison to surface sampling”
- “The airborne contagiousness of respiratory viruses: A comparative analysis and implications for mitigation”
- “Age-dependent regulation of SARS-CoV-2 cell entry genes and cell death programs correlates with COVID-19 severity”
📖 Let’s get started…
This study, “Spread of SARS-CoV-2 in Hospital Areas,” was published on September 20, 2021. The researchers performed a systematic sampling and analysis of airborne COVID-19 RNA in different hospital areas to assess the viral spread. It was found that the highest occurrence of SARS-CoV-2 was found in two related units at hospitals. Rooms with COVID-19 patients receiving regular care and the adjacent hallways.
➡️ Interestingly, ICU units treating COVID infected patients had a rate of airborne SARS-CoV-2 ten times lower –– why?
Patients in ICU for COVID-19 were intubated, connected to respirators that filtered all exhaled air and prevented the virus from being released into the air. In areas of the hospitals treating COVID-19 patients that were not intubated, researchers believe that the ventilation systems were responsible for transferring the airborne virus to other rooms and hallway corridors.
🔍 Study Highlights
“Systematic air filtration was performed in rooms with COVID-19 infected patients, in corridors adjacent to these rooms, to rooms of intensive care units, and to rooms with infected and uninfected patients, and in open spaces. RNA was extracted from the filters, and real-time reverse transcription polymerase chain reaction was performed using the LightMix Modular SARS-CoV-2 E-gene.”
“The highest occurrence of RNA was found in the rooms with COVID-19 patients (mean 2600 c/m3) and the adjacent corridor (mean 4000 c/m3), which was statistically significantly more exposed (p < 0.01). This difference was related to the ventilation systems. As is commonly found in many hospitals, each of the rooms had an individual air inlet and outlet, while in the corridors these devices were located at the distance of every four rooms. There was a significant transfer of viruses from the COVID-19 patients’ rooms to the corridors.”
“The airborne SARS-CoV-2 RNA in the corridors of ICUs with COVID-19 patients or care rooms of uninfected patients were ten times lower, averages 190 c/m3 and 180 c/m3, respectively, without presenting significant differences. In all COVID-19 ICU rooms, patients were intubated and connected to respirators that filtered all exhaled air and prevented virus release, resulting in significantly lower viral concentrations in adjacent corridors.”
“The results show that the greatest risk of nosocomial infection may also occur in hospital areas not directly exposed to the exhaled breath of infected patients. Hospitals should evaluate the ventilation systems of all units to minimize possible contagion and, most importantly, direct monitoring of SARS-CoV-2 in the air should be carried out to prevent unexpected viral exposures.”
This study, “Airborne SARS-CoV-2 Surveillance in Hospital Environment Using High-Flowrate Air Samplers and its Comparison to Surface Sampling,” was published September 14, 2021. The researchers were seeking reliable methods to detect COVID-19 at large gatherings or events using air monitors. A hospital setting with infected COVID-19 patients was examined for their sample environment. This study tested the feasibility of using high-flow rate air samplers utilizing RNA extraction. They also compared those samples with detecting the presence of SARS-CoV-2 using surface swab samples collected nearby.
The setting for this study was in a University Hospital in Singapore housing COVID-19 patients between February to May 2020. Two wards were analyzed –– a naturally ventilated isolation unit and a mechanically ventilated isolation unit. Interestingly, despite the difference in ventilation between the two different wards, the airborne SARS-CoV-2 detection was comparable with a 60%-87.5% success rate.
Researchers of this study also revealed what many have already suspected. Be careful when using communal restrooms. The novel coronavirus was noticeably prevalent in both surface areas and the air in bathrooms at this hospital. However, overall, they found that out of 73 surfaces sampled, only seven were contaminated. Covid-19 is much less likely to be found on surface areas, confirming that this virus is most often transferred while airborne.
🔍 Study Highlights
“Reliable methods to detect the presence of SARS-CoV-2 at venues where people gather are essential for epidemiological surveillance to guide public policy. Communal screening of air in a highly crowded space has the potential to provide early warning on the presence and potential transmission of SARS-CoV-2, as suggested by studies early in the epidemic. As hospitals and public facilities apply varying degrees of restrictions and regulations, it is important to provide multiple methodological options to enable environmental SARS-CoV-2 surveillance under different conditions.”
“A robust surveillance method for the presence of SARS-CoV-2 in the environment is essential to allow safe resumption of normal activities, given that it could require multiple years to achieve reasonable vaccination rate globally. There is also no assurance that vaccines will eliminate the need for continued physical distancing.”
“A large-scale and accurate test regime that could detect the virus will aid in designing practical screening strategies. Currently, SARS-CoV-2 testing is performed on an individual level, which has proven to be laborious and costly.”
“Interestingly, despite the higher positive rate of the isolation ward air samples, the open ward air samples had an averagely higher viral load (Figure 1d). In addition to the abovementioned distance factor, differences in ventilation scheme between the two wards (mechanical vs. natural ventilation) as well as variabilities associated with the patients that were present at the time of sampling are the likely confounding factors for the observed dynamics between the investigated wards.”
This study, “The Airborne Contagiousness of Respiratory Viruses: A Comparative analysis and implications for mitigation,” was published on August 11, 2021, and tackles the question of the strength a predictive estimation approach offers to compare the contagiousness of different respiratory pathogens. We found this study particularly interesting –– how do various respiratory pathogens compare?
➡️ The researchers compared the transmissibility of SARS-CoV-1, SARS-CoV-2, MERS, Measles virus, Adenovirus, Rhinovirus, Coxsackievirus, Seasonal Influenza virus, and Mycobacterium Tuberculosis (TB).
The current Pandemic has renewed interest in the study of airborne contagions in indoor environments. Airborne transmission of respiratory tract infection is caused by an infected person emitting droplets while breathing, speaking, or sneezing.
This transmissability is why the use of masks to mitigate SARS-CoV-2 is so essential. The inhalation of microscopic virus or bacteria-laden droplet nuclei is all it takes to become infected yourself. Did you know that the viral loads of the Delta variant of the novel coronavirus are 1,000 times higher on the first day of positive testing than those of the original strains studied in 2020?
➡️ The Measles virus was found to be the most infectious out of all of the different airborne pathogens examined in this study.
🔍 Study Highlights
“The aim of this work is threefold: (i) to assess the strength of the predictive estimation approach for the airborne emission rate of common respiratory pathogens by comparing estimates to back-calculated values reported in literature, (ii) to use the estimates to compare the contagiousness of the modeled pathogens through the airborne route, and (iii) to assess the ability of modern standards of ventilation to prevent their epidemic spread.”
“We applied the approach to SARS-CoV-1, SARS-CoV-2, MERS, measles virus, adenovirus, rhinovirus, coxsackievirus, seasonal influenza virus and Mycobacterium tuberculosis (TB) and compared quanta emission rate (ERq) estimates to literature values. We calculated infection risk in a prototypical classroom and barracks to assess the relative ability of ventilation to mitigate airborne transmission.”
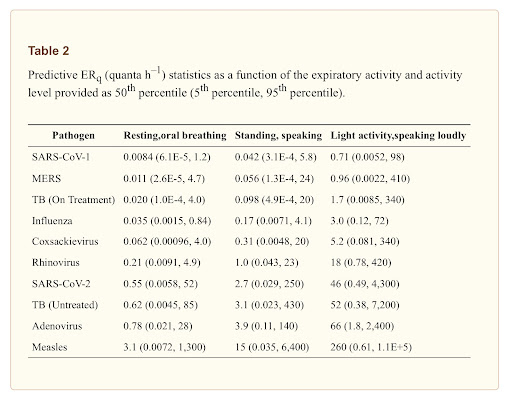
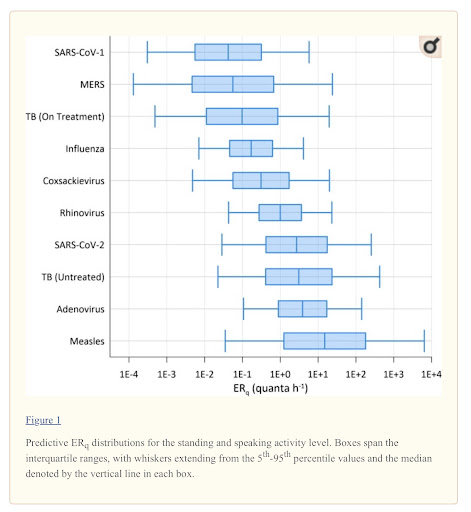
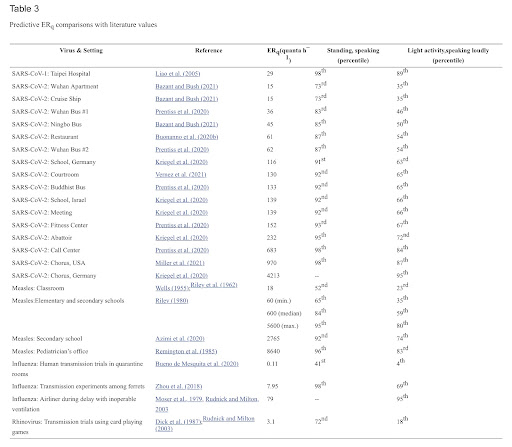
“The predictive estimation approach advances methods of prospective risk assessment for airborne transmission of disease. Our calculations suggest measles virus to be the most contagious of those evaluated, but the median estimates for SARS-CoV-2, adenovirus, and untreated, active TB are within the same order of magnitude.”
“Our risk modeling scenarios for a classroom and barracks show that even a high ventilation rate of 14 L s–1 p–1 will likely fail to prevent the spread of adenovirus, TB (untreated), SARS-CoV-2 and measles in a fully susceptible population, indicating that additional engineering controls such as advanced ventilation design or air disinfection are necessary to supplement public health measures.”
“For SARS-CoV-2, our results highlight the importance of masking for both source control and personal respiratory protection and reinforce the need for airborne precaution and/or isolation rooms in health care or cohabitation settings treating COVID-19 patients.”
This study, “Age-Dependent Regulation of SARS-CoV-2 Cell Entry Genes and Cell Death Programs Correlates with COVID-19 Severity,” was published on August 18, 2021. The researchers seek to answer the question plaguing all of us –– why does this novel coronavirus generally affect age groups so differently?
➡️ The researchers investigated how the expression of viral entry & cell death genes in the lungs of hosts could vary in different age groups. Also, they questioned how this variation relates to disease severity.
The reason for this difference was unclear until the researchers of this study discovered that the protein expression of the enzyme that results in the cell entry receptor for SARS-CoV-2 increases drastically with advancing age. Also, notably, infants infected often have a difficult time and higher hospitalization rates than older children.
🔍 Study Highlights
“The causal agent for COVID-19, the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), infects host cells through interaction with the cell surface proteins angiotensin-converting enzyme 2 (ACE2) and transmembrane serine protease 2 (TMPRSS2) (5). For SARS-CoV-2 infection to occur, the spike protein of a viral particle must bind ACE2 and undergo cleavage by TMPRSS2 to allow the particle to fuse with the host plasma membrane and gain entry into the cell (5).”
“Viral entry represents just one of several steps in COVID-19 pathogenesis, each of which involves genes that may potentially be developmentally regulated in the lung and elsewhere. Apoptotic and nonapoptotic host cell death pathways also play important roles, as they can modulate disease pathogenesis after viral infection (12, 13). Specifically, coronavirus-infected cells typically experience endoplasmic reticulum (ER) stress due to the intensive production of infectious virions and activate the unfolded protein response (UPR) to adapt to this stress (14).”
“Thus, across a diverse set of microarray datasets, lung Ace2 levels were relatively high immediately after birth, significantly lower during adolescence, and increased in adulthood, reaching their peak at advanced age.”
“The shifting expression of ACE2 in the mouse lung suggested that regulation in human lung tissue may also be dynamic.”
“Our results, thus far, suggested that higher lung ACE2 expression in infancy and late adulthood would broaden the pool of cells that can be potentially infected by SARS-CoV-2 at those ages. At all ages, however, ACE2 expression was highly variable between individuals, indicating that humans of any age can potentially be infected by SARS-CoV-2 throughout the respiratory system.”
“Our studies provide evidence that ACE2, TMPRSS2, and apoptotic programs are dynamically regulated by age and cell type in the lung and correlate with severity of COVID-19 disease.”
“Furthermore, the strong up-regulation of ACE2 in late adulthood across multiple cell types in the lung increases the number of cells that can potentially be infected by SARS-CoV-2.”
“Our findings also suggest a potential therapeutic approach focused on cell death responses to infection, wherein apoptotic priming in adult lung tissue would be modulated to match that in pediatric lung. This approach, which could involve administration of BH3 mimetics [small-molecule BCL-2 family inhibitors (44)] systemically or directly to lung tissue via inhalation, would be expected to reduce virus replication in adults as infected, stressed cells would undergo apoptosis earlier, halting further virion production.”
“Initial infection and cell death are only two of an extensive set of factors influencing disease course in patients with COVID-19 and other respiratory viruses. Immune response (35, 62), host genetics (63), environmental factors (8), and therapeutic interventions (64, 65) may all play contributing roles in determining infection outcome.”
Met One Instruments, Inc. offers air quality monitors for cleanrooms and controlled environments, which act as a control for experiments such as the studies we have highlighted here. Cleanrooms are closed, controlled environments utilizing environmental parameter control and filtration systems to purify the inside air, commonly for manufacturing and research. These environments require that stringent cleanliness, temperature, and moisture levels are maintained, ensuring contamination-free environments.
Our BT-620 Benchtop Particle Counter, now CE certified, is seamlessly integrated into existing facility software and building automation systems providing the highest level of data integrity to ensure a contamination-free cleanroom. Our portable units, such as the DR-528, offer multiple size selections and significant data storage when monitoring air in auxiliary areas.
Hospitals, surgery centers, clinics, and medical laboratories monitor air filtration systems for microorganisms and particles to protect the health and wellness of patients, such as the ICU ventilation protocols employed for COVID-19 units. The GT-324 is well-suited for indoor air monitoring where HVAC and Filter Performance follow ISO16890 and ASHRAE 52.2 standards. Our cleanroom instrumentation offers the precision and data integrity needed in these high-stakes environments.
➡️ Thank you for joining us for Part-Two of our series for Indoor Air Quality Month 2021!
We hope that this examination of independent IAQ studies about COVID-19 was interesting and helpful to you. The Air Monitoring equipment that we produce here in Grants Pass, Oregon, at Met One Instruments, Inc. is often chosen to act as a control in studies like these. We have been proud to be considered the standard in precision air quality measurement, whether outdoors or indoors, since 1989.
💡 Would you like to learn more?
🔗 Read Our Article –– IAQ 101: Everything You Need to Know About Indoor Air Quality

🔗 Read Our Article –– Learn Why the EPA Choose Met One Instruments BAM 1020 FEM PM2.5 to Test the Reliability of New Handheld Air Monitors on the Market




